TL98 : OnkoSight Advanced NGS MDS Panel
INFORMATION:
Alternate Name:
OSA NGS MDS
Methodology:
Genotyping by Next Generation Sequencing
Clinical Utility:
Tumor sequencing by NGS detecting mutations in 18 genes associated with MDS. Mutations often have prognostic significance, may confer resistance to FDA approved therapies, or may qualify patients for late-stage clinical trials.
Gene content:
ASXL1
BCOR
CBL
DNMT3A
ETV6
EZH2
JAK2
KRAS
NRAS
PTPN11
RUNX1
SETBP1
SF3B1
SRSF2
TET2
TP53
U2AF1
ZRSR2
ORDERING:
Test Code:
TL98-8
Turnaround Time:
10 DAYS
Preferred Specimen:
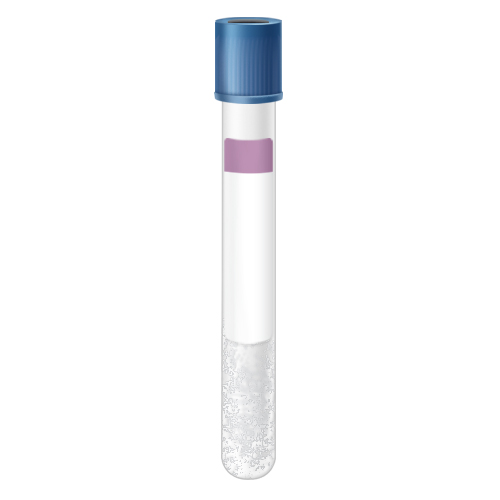
2 mL Peripheral Blood - Lavender Top
Collection:
| Container | Qty | Temp | Stability |
|---|---|---|---|
| Peripheral Blood - Lavender Top | 1 | Room Temp | 30 DAYS |
Collection Instructions:
LPB: Fill tube, invert gently 5-6 times, label with patient's name
Storage Transport Instructions:
BML/BMG: Place bone marrow into tube, label with patient's name and send in provided kit.
LPB/GPB: Place peripheral blood into tube, label with patient's name and send in provided kit.
Alternative specimen: Extracted DNA is acceptable, providing that the isolation of nucleic acid occurs in a CLIA-certified laboratory or a laboratory meeting equivalent requirements as determined by CMS and/or the CAP.
Storage/Transport Instruction: Specimen can be either RT or refrigerated
LPB/GPB: Place peripheral blood into tube, label with patient's name and send in provided kit.
Alternative specimen: Extracted DNA is acceptable, providing that the isolation of nucleic acid occurs in a CLIA-certified laboratory or a laboratory meeting equivalent requirements as determined by CMS and/or the CAP.
Storage/Transport Instruction: Specimen can be either RT or refrigerated
Specimen Comment:
Decalcified specimens are not recommended for NGS testing.
Alternative Specimen:
Peripheral Blood - Green Top, Bone Marrow - Green Top, Bone Marrow - Lavender Top, Eppendorf tube, Formalin-fixed, Paraffin-embedded Tissue, Unstained Slide
Billing:
CPT Codes:
81450 x 1
CPT Code Disclaimer
CPT codes provided are for informational purposes only. Accuracy of CPT presented should be validated prior to consideration for billing.
CPT coding is the sole responsibility of the billing party. Please direct any questions regarding CPT coding to the payer being billed.

 Back to Test Directory
Back to Test Directory